
You must be a medical professional to view these pages!

You must be a medical professional to view these pages!

An Innovative Pulmonary Surfactant Delivery Solution for Neonates with NRDS.
BLEScath®– Designed by clinical experts who understand the practice and importance of delivering exogenous pulmonary surfactant less invasively to neonates diagnosed with neonatal respiratory distress syndrome (NRDS).
Proudly Made In Canada
Download IFU

NRDS Rescue Treatment calls for an integrated scientific solution.
BLEScath® is intended for the administration of pulmonary surfactant, such as bovine lipid extract surfactant suspension, BLES® using the less invasive surfactant administration (LISA) technique or minimally invasive surfactant therapy (MIST) for rescue treatment of NRDS.
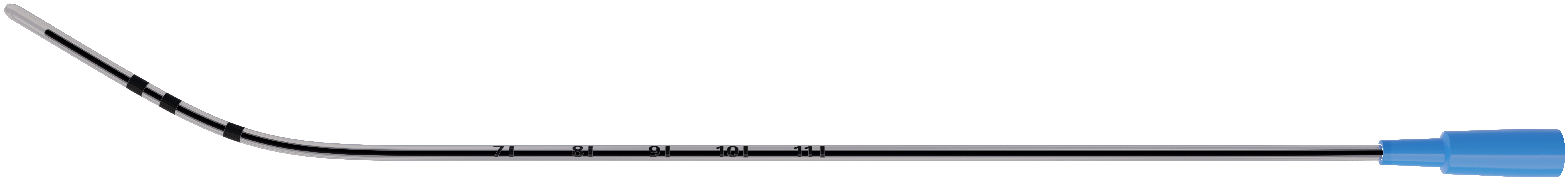

Smart engineering supported by clinical research.
Surfactant researchers developed BLEScath®. This catheter has an integrated stainless steel stylet that is rigid but flexible for ease of insertion. The device can be bent and held into the preferred shape, providing a balance between maneuverability and procedural success without the need for Magill forceps.
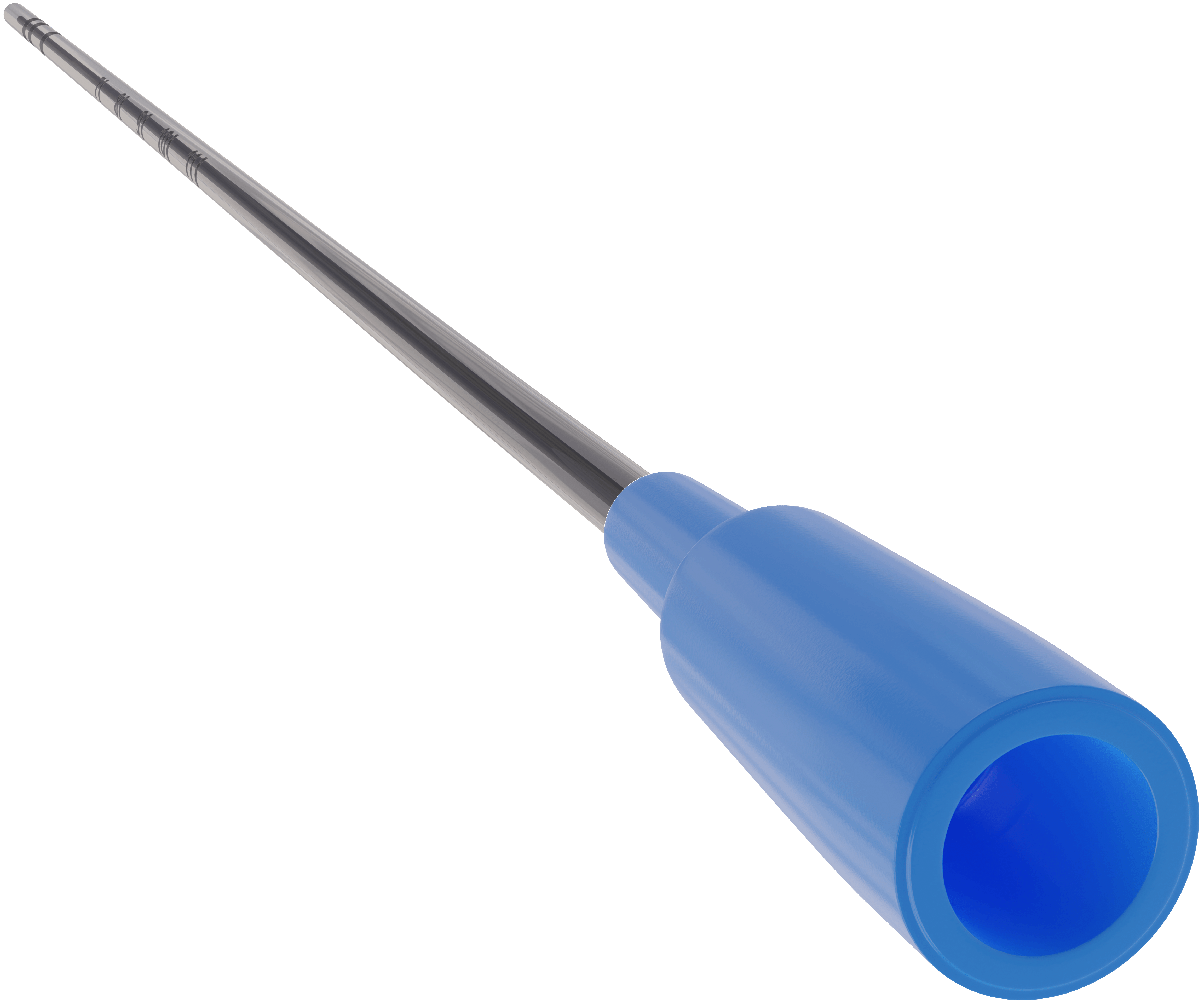

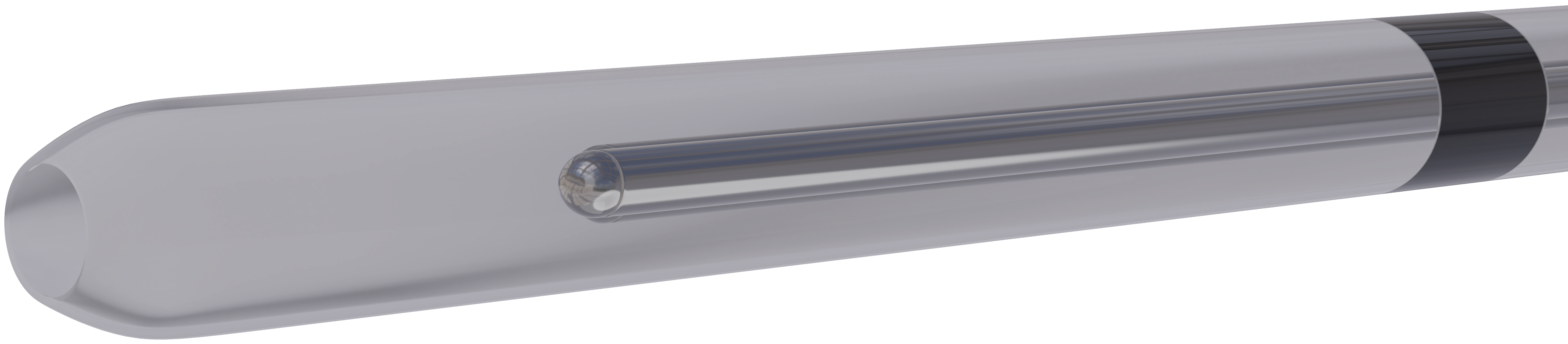
Precision and neonatal safety are pivotal.
The stylet is fixed and anchored within the catheter. Markings that indicate insertion depth and tip to lip distance coupled with a soft, rounded distal tip address tracheal insertion parameters and may minimize associated procedural soft tissue injury.

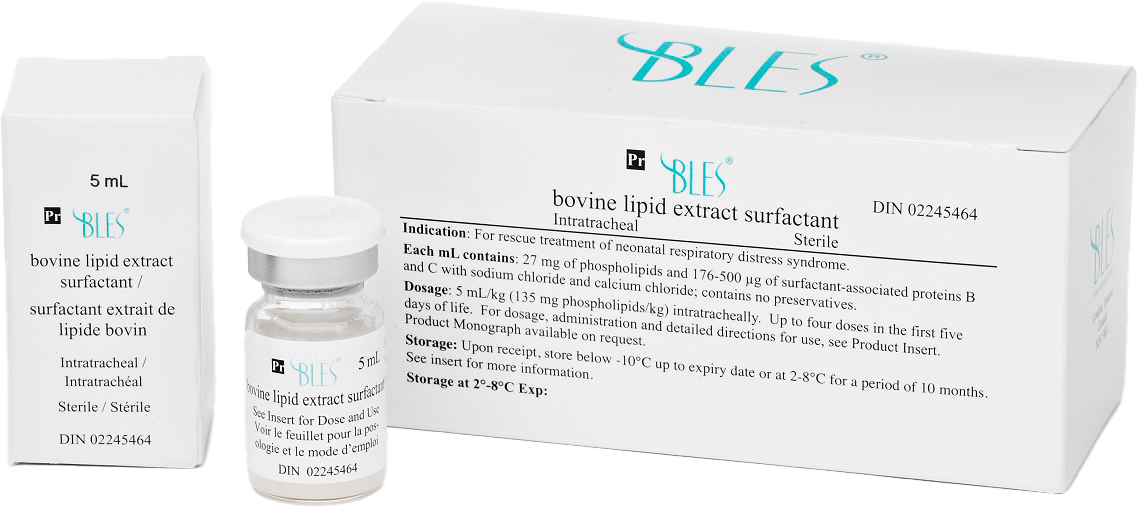
Leaders lead and so do we!
#1 Leading Pulmonary Surfactant Solution in Canada.
BLES® is recognized as the treatment of choice for NRDS in Canada. Most NICUs selectively chose BLES® as an effective solution to combat the collapse of lung tissue at alveoli level1. BLES® (bovine lipid extract surfactant suspension) is indicated for: rescue treatment of Neonatal Respiratory Distress Syndrome (NRDS) and is compatible with BLEScath®, a purpose-built thin catheter specifically used for LISA in premature infants suffering from NRDS.
1. Lemyre B, Lacaze-Masmonteil T, Shah PS, Bodani J, Doucette S, Dunn M, et al. Poractant alfa versus bovine lipid extract surfactant: Prospective Comparative Effectiveness Study. Journal of Perinatology. 2022;
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |